Carbon In Water And Espresso Extract – The contents of the previous part are merged in this section to evaluate the influence of varied water compositions on extraction.
This entails determining the beginning water composition, selecting a target, and selecting an appropriate water treatment technology to achieve the desired composition change. Let’s find out with Helena.
Cupping with a Variety of Waters
A cupping experiment explored the impact of water composition on the sensory qualities of coffee extraction (Wellinger and Yeretzian, 2015).
Three different water compositions were created in this experiment utilizing a decarbonizer-type ion exchanger, which reduces overall hardness and alkalinity in equal amounts.
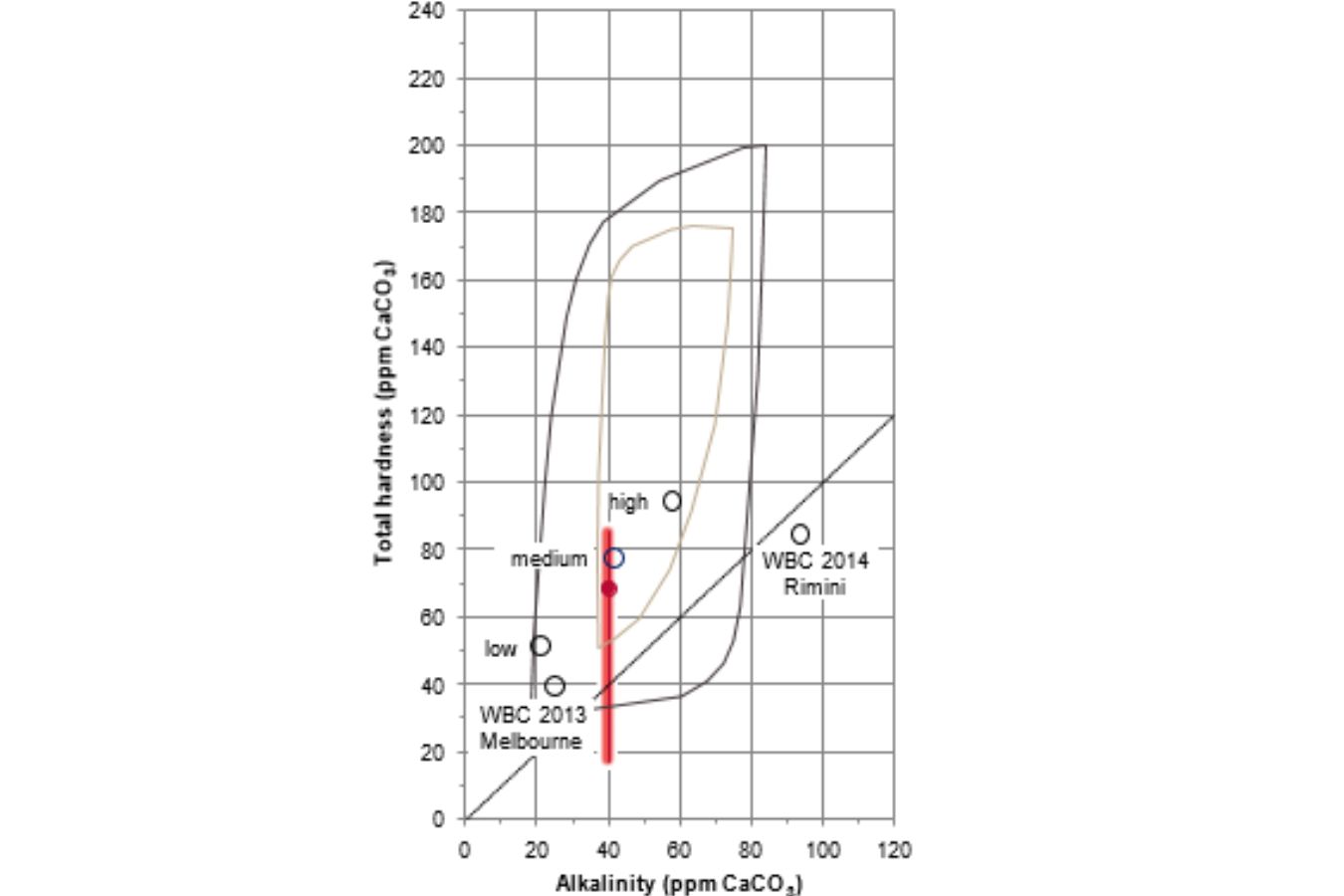
Figure 16.5 shows the water compositions achieved, with the alkalinity altered by 50% compared to the SCAA objective of 40 ppm CaCO3 alkalinity.
The water compositions used in the World Barista Championships (WBC) in Melbourne in 2013 and Rimini in 2014 are also shown for reference (data from Colonna-Dashwood and Hendon, 2015). The following two coffees were utilized in the experiment:
- La Argelia farm, Tolima area, 1580e1900 m, Colombia, the washing process, Caturra and Castillo, screen size 15,
- Lagoa Formosa farm, Minas Gerais region, 1000e1200 m, natural process, yellow bourbon, Tupi, Icatu, and Yellow actual, screen size 16e18.
In a blind tasting session, the coffees were prepared using the SCAA cupping protocol with minor changes to the sensory attributes: At 93°C, 13.8 g of freshly ground coffee was combined with 250 mL water. Scores for fragrance, homogeneity, and clean cup were not considered. On a scale of 1e10, the sweetness was assessed.
The findings reveal that even minor variations in hardness and alkalinity significantly impact coffee’s sensory qualities.
Although more research is needed to understand the mechanisms causing these attribute changes fully, we believe it is the result of a combination of two effects:
(1) overall hardness impacting extraction efficiency and (2) alkalinity increasing perceived acidity.
In conclusion, the findings show that, while general trends can be formed for how a change in hardness or alkalinity affects the sensory qualities of a coffee beverage, there are variances amongst coffees that are intimately tied to their entire flavor profile.
The fact that low mineral content water received the highest score for most attributes could be due to the relatively high extraction yield achieved by the cupping method (due to extended contact time compared to other brewing methods), which also results in a heavier body perception than drip coffee.
As a result, if the same coffees were used but a different extraction process, such as a filter, the results could be drastically different.
Decarbonization of water and extraction of espresso
When using a decarbonizer to treat hard water in espresso machines, one of the most typical issues is the development of carbonic acid, which results in excess dissolved carbon dioxide in the water.
The protons released in exchange for Ca2 and Mg2 neutralize the hydrogen carbonate in the water, generating carbonic acid, which, depending on pressure and temperature, can decrease as carbon dioxide.
Carbonic acid cannot escape as carbon dioxide in a cafe’ where the water from the ion exchange cartridge is pressured up to the espresso machine, which can substantially impact the extraction.
Carbon dioxide becomes more soluble as pressure rises, and as the temperature increases, it becomes less soluble. When high-carbonic-acid water enters the extraction chamber (basket), it provides an additional resistance (see Section 4) in the same manner as carbon dioxide from newly roasted and ground coffee does (see Section 4).
As a result, when hard water is treated with a decarbonizer, an effect similar to that seen in espresso extraction when coffee is newly roasted before extraction may be noticed.
Freshly roasted coffee produces a considerable amount of crema with enormous bubbles that collapse faster than coffee that has been rested for a more extended period.
For example, lowering the alkalinity by 200 ppm CaCO3 alkalinity will result in an increase of 176 mg/L in dissolved carbon dioxide. As a result, if we extract a double espresso with 15 g of Arabica coffee that has been freshly ground (2 min) within one h after roasting using 30 g of decarbonized water, the water will add 20% to the carbon dioxide already present in the coffee grounds.
Degassing, a well-established technique in other industrial applications, might remove the excess carbon dioxide created by decarbonization.
Alternatively, the water could be treated with a demineralized, eliminating all ions without adding further dissolved carbon dioxide.