
Effect Of Alkalinity On Coffee Extract: In the analysis of SCA water quality standards, we know that Alkalinity is the ability of water to buffer acids, i.e., the power of water to neutralize acids.
And under standard conditions, Alkalinity is limited to about 40 mg/l; With this simple approach, much of the “powerful properties” of Alkalinity for coffee extracts remain unclear. Alkalinity can help stabilize the flavor of the coffee, but it can also level out all the acidity we want.
Therefore, in this article, Helena will provide another aspect of water quality for coffee making, how Alkalinity is so essential, and suggestions for fine-tuning the Alkalinity so that Suitable for Pour-over or Espresso techniques.
Alkalinity is a term invented by oceanographers (Professor Wilhelm Dittmar), but hydrologists also use it to describe the temporary hardness of water (carbonate hardness). On the other hand, Alkalinity is confused with something else in chemistry. Not everyone who loves coffee is also interested in learning chemistry, so you can quickly go through the following three paragraphs.

Total Alkalinity is usually limited by pH, but they are not, while pH is a measure of the ratio (logarithmic) of OH – free ions to H + ions in solution; With pH = 7, we have neutral water, when there are many H+ ions we will have an acidic solution (pH<7), in contrast to a more significant amount of OH- ions we have a basic solution (pH>7). This is why total Alkalinity is often confused with lye (or basic explanation).
Going back to Alkalinity, it is measured by the amount of HCO3– ions in the water, which can capture any free H+ ions added to the solution and prevent them from making the solution more acidic by forming carbonic acid (H2CO3), like the familiar diagram in 10th grade that we learned here:
H + + HCO3 – H 2 CO 3
For this reason, HCO3- is called an alkaline buffer. In coffww extract HCO3- can react with aromatic acids extracted from coffee beans, so SCA recommends a total alkalinity range between 40 ppm as CaCO3.
Why is Alkalinity more critical than pH?
Even when raindrops fell to the earth, gaseous compounds diffused into it, most notably CO2, NO2, SO 2 … In which dissolved CO2 combined with water to form Carbonic acid made for rainwater always be acidic at about pH 5.5.
However, it should not be confused with acid rain in areas where the air is polluted with sulfur and nitrous oxide, which causes rainwater to have a pH of 4.5 or less.

But why do we have to talk about acidity in water? Why not simply comply with SCA’s standards?
That’s because acid rain is the perfect example to demonstrate the power of Alkalinity over pH. One liter of average tap water with an alkalinity of 150 ppm CaCO3 can neutralize 100 liters of acidic rainwater at pH 4.5.
Making the same deduction with water and coffee, the effect of Alkalinity is hundreds of times higher than pH, informing the final acidity of the coffee extract, so the second half of this article, we will focus on clarifying the unfinished business on the pH scale – That’s why alkaline water is better than acidic water and why you should adjust water quality according to ratio or extraction method.
Average tap water is alkaline in the range of 150 ppm CaCO3* and has a total hardness of about 170 ppm CaCO3 – This is considered medium hard water. This allows tap water to neutralize hundreds of liters of rainwater.
(*) At this point, you might be thinking, “How the hell does CaCO3 go with the weird metric ‘ppm.’ Chemists love to create exotic units of measurement, and this is a prime example. ppm CaCO3 is interpreted as “parts per million CaCO3” (see also 10th-grade chemistry textbook). But because CaCO3 is also used as a unit of measurement for water hardness, it is often confused with Alkalinity.
Illustrate the ‘buffer’ of Alkalinity
For example, a wooden bench and a stone bench lie along the path in a sunny park. The wooden chair doesn’t feel too hot, even with the sun shining all day long, but on the contrary, the stone bench – which is in the same situation – will be ready to roast your butt.
If you measure both chairs with an infrared thermometer, you can see that the stone bench has a large amount of heat stored (because the specific heat capacity of stone is much greater than that of wood). The stone bench – for the same reason – will stay warm much longer after the sun goes down.
With benches in the park, you can easily decide where to sit by seeing if it is stone or not. Meanwhile, with water, the pH only shows the current state of the water, but it does not tell us how the pH will change when combined with coffee.
This change is highly dependent on the Alkalinity of the water. With water ‘soaked’ with a large amount of alkali (like a stone bench that stores a lot of heat), the acids in the coffee will be significantly neutralized. So Alkalinity provides a hint for you to predict how much flavor your coffee will lose when combined with water.
In fact, the acidity you get from a given cup of coffee corresponds to the amount of acid extracted from the coffee minus the amount of acid that has neutralized the alkali in the water.
Meaning of alkaline buffer in coffee extract
Understanding the Alkalinity and acidity in water gives you the foundation to understand how water and coffee interact to form the acidic character of the extract. There are two factors you need to pay attention to.
First is the Total Alkalinity in the water. The more alkaline the water, the higher its ability to neutralize the natural acids in coffee (citric acid, Quinic Acid, Chlorogenic Acid, etc.). The acidity itself is still there; we don’t feel it in the taste, only it’s called “flat taste.”

The second factor is the percentage of water in your coffee extraction. A cup of Ristretto usually has a coffee ratio of ÷ extraction = 1÷1 or 1÷1.5 – Whereas, with the Pour Over technique, this ratio goes up to 1÷15. This means that for an espresso concentrate, only 10% of the Alkalinity is available to neutralize some natural acidity.
This is a matter to consider – because, with the same coffee and water, you’ll notice more bite from the Espresso than with a pour-over that’s neutralized ten times more alkali.
Extended inference
Continuing to follow this logic (as well as the experimental studies of the SCA Standards Committee), the range of total Alkalinity in water may have to open to a new frontier, instead of being limited to around 40mg/l, Alkalinity in water when brewing Espresso can range between 250 and 450 mg/l; That is, the current total Alkalinity should be multiplied by 7.3 (corresponding to the coefficient between a cup of Espresso (water/coffee ratio = 2) versus standard drip coffee (water/coffee ratio = 14). ,6).
Studies from SCA show that flavors in coffee tend to be significantly neutralized when high total hardness water (>250 ppm CaCO3) is used and vice versa when low total hardness water is used (<40 ppm). CaCO3) taste is almost unaffected.
However, tests also show that the effect of total hardness is unlikely to affect the overall efficiency of the extraction (20 to 250 ppm CaCO3) – no matter how hard the water is. Controls how much soluble coffee you extract.
Understanding the Alkalinity and acidity in water gives you the foundation to understand how water and coffee interact to form the acidic character of the extract. There are two factors you need to pay attention to
First is the Total Alkalinity in the water. The more alkaline the water, the higher its ability to neutralize the natural acids in coffee (citric acid, Quinic Acid, Chlorogenic Acid, etc.). The acidity itself is still there; we don’t feel it in the taste, only in this case it’s called “flat taste.”
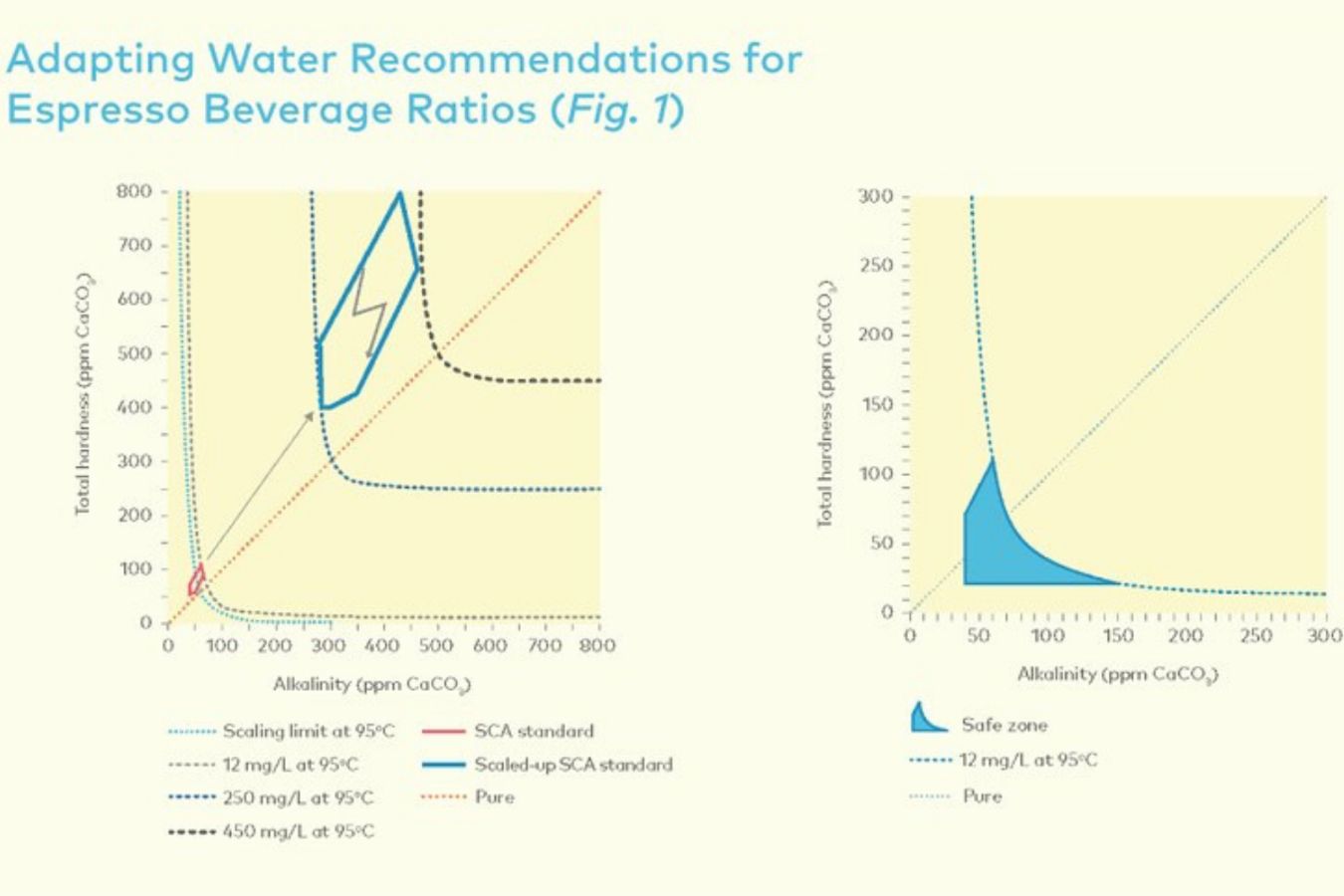
However, this puts the water alkalinity standard when brewing Espresso into the “danger” zone with the potential for highly high scale formation (imagine 0.25–0.45g salt per liter of water), ingredients in water lead to heavy deposits or corrosion of your machinery.
This article outlines some key insights from the SCA coffee brewing standards manual. With the topic Water and Coffee Acidity: How to Adapt Your Water for Different Extraction Methods, including the research of professor DR. MARCO WELLinger – A coffee researcher in chemistry, technology, and sensory analysis at the Institute of Chemistry and Biotechnology at ZHAW Wädenswil.
References:
- Primecoffea, Tác động của độ kiềm đến chiết xuất cà phê, Tháng bảy 6, 2020
