
Stability And Crema Formation?
Foam is a complicated and challenging process that involves four main events: bubble production, bubble rise, drainage, and coalescence and disproportionation (Bamforth, 2004). Foam is a coarse dispersion of gas bubbles in a continuous liquid phase.
The CO2 produced during coffee roasting, which is partially imprisoned within the cell structure, makes up the majority of the gas phase in espresso coffee.
This continuous phase is an oil-in-water emulsion of microscopic oil droplets (less than 10 mm) suspended in an aqueous solution containing numerous coffee ingredients (such as sugars, acids, and proteins) as well as minute solid coffee cell-wall fragments (2e5 mm) (Illy and Viani, 2005). The bubble has a diameter of around 100 mm and looks to be covered in protein-rich material.
Espresso crema is categorized as a metastable froth with a defined lifespan (Dickinson, 1992). In most circumstances, it can take up to 40 minutes for the crema to vanish (Dalla Rosa et al., 1986).
The qualities of the crema change as it ages, from a fine liquid froth in freshly produced espresso to a dry polyhedral foam. Ideally, the crema should account for at least 10% of the total weight of the dish.
Espresso crema is categorized as a metastable froth with a defined lifespan (Dickinson, 1992). In most circumstances, it can take up to 40 minutes for the crema to vanish (Dalla Rosa et al., 1986).
The qualities of the crema change as it ages, from a fine liquid froth in freshly produced espresso to a dry polyhedral foam. With a foam density of 0.30e0.50 g/mL, the crema should make up at least 10% of the volume of an espresso (Illy and Viani, 2005). (Navarini et al., 2006).
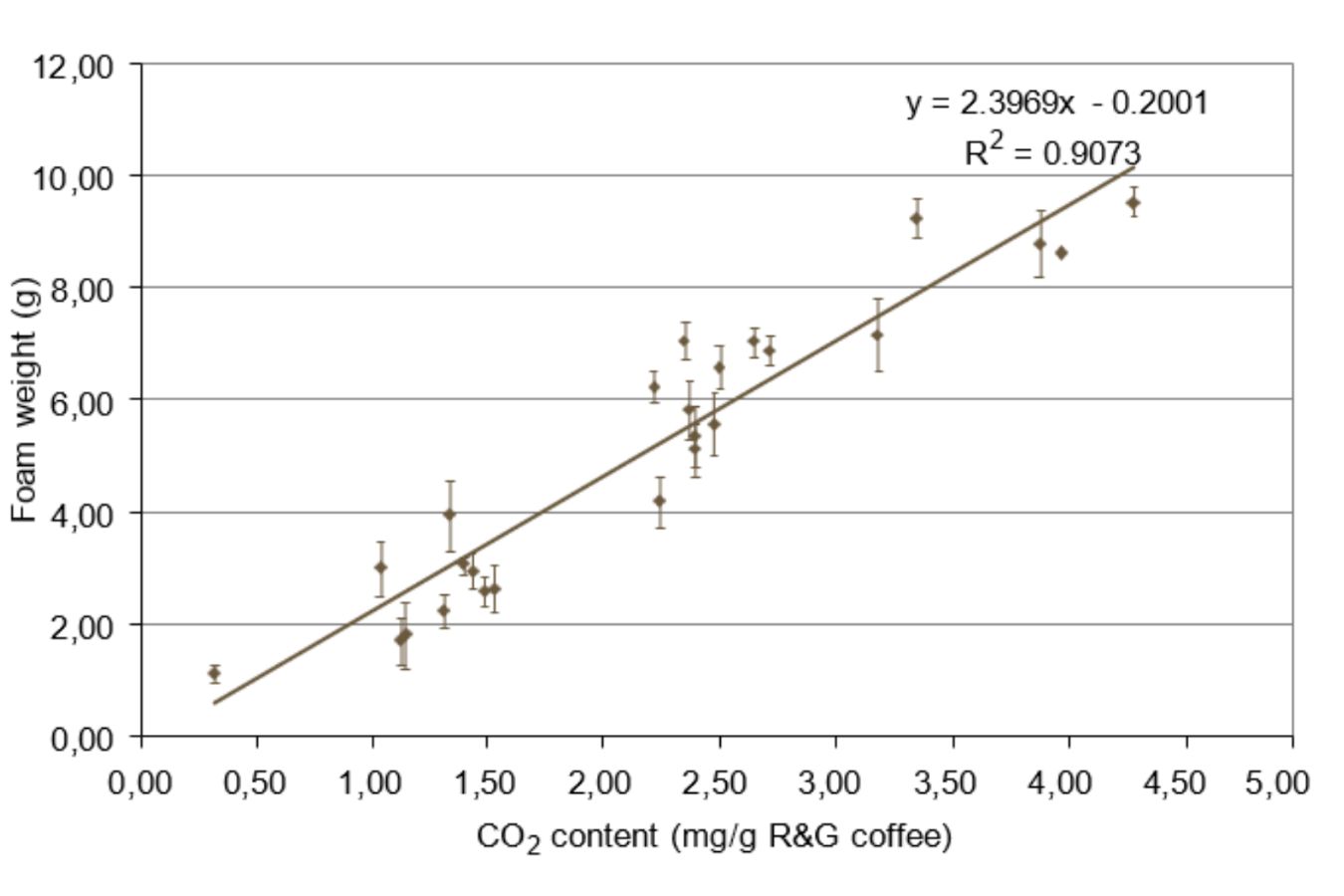
The latter study’s authors discovered a linear association between CO2 content and foam weight and volume.
Several attempts have been made to explain how crema forms in espresso coffee. The coffee oils are emulsified into the extracted liquid as water is pushed through the coffee under pressure.
Furthermore, roasted coffee emits CO2 for a short period (degassing), and the longer the coffee is exposed to ambient pressure, the more CO2 it emits.
This is why some espresso packaging materials are designed to keep overpressure and CO2 out of the coffee matrix. During extraction, excess CO2 is released.
Even though CO2 has been suggested as the gas phase responsible for espresso coffee foaming, coffee scientists have yet to examine the bubble formation mechanism thoroughly.
On the other hand, the relationship between CO2 chemistry and foam generation has been investigated. Indeed, research reveals that the bicarbonate carbonic acid equilibrium is involved in the dynamics of the espresso extraction’s brief phase (Fond, 1995, Chapter 16).
The initial wetting step of brewing espresso, when hot water seeps into the spaces in coffee particles and intended intraparticle gas are concurrently driven out of the coffee bed, is known as the transient phase of extraction (Petracco and Liverani, 1993).
This mass transfer between coffee particles and water happens simultaneously, and the bicarbonate ions in the extraction fluid help speed up the process.
Chemical reactions occur at high temperatures in the coffee bed due to the displacement of its equilibrium due to the pH evolution during brewing (from 7.0 to 7.5 to 5.5e5.0).
The coffee is compacted by water pressure, the swelling of the coffee grinds, and CO2 degassing, which results in the foam and emulsion that gives espresso its much-loved crema.
According to other research, CO2 supersaturation conditions in coffee may be a driving reason for creating espresso coffee froth (Navarini et al., 2006).
More specifically, CO2 solubilization (which occurs in the coffee bed) in high-pressure, high-temperature water may result in supersaturation conditions in the final cup, leading to the nucleation of tiny bubbles.
To elaborate, during CO2 extraction at high pressure and at a water temperature of 100°C, the CO2 concentration in water may be lower than the CO2 solubility. At 1 bar and 70°C, however, it may be above solubility.
When there is heterogeneous nucleation and bubble rise following a phase transition (from high pressure to ambient pressure) when the high-pressurized water leaves the coffee bed and enters the cup, these conditions are compatible with foaming.
Champagne also exhibits this phenomenon, which is known as effervescence. In this scenario, effervescence could refer to the effect that occurs right after the espresso is prepared.
Espresso’s micronic solid particles and submicronic cell-wall fragments may act as nucleation sites. Furthermore, due to the small volume of an espresso beverage, bubble rise is limited to around 1.5e2 cm, resulting in distinctively tiny bubbles in espresso foam (Illy and Navarini, 2011).
Foam instability typically occurs due to three occurrences once the foam has been produced (Prins, 1988). When the layer separating the bubbles collapses, there is first a coalescence among the bubbles.
The second ripening type is Ostwald ripening, which occurs when the foam sizes are polydispersed. Pressure differences between various-sized bubbles disperse the smaller bubbles into the larger ones during this phase.
Finally, gravity pulls the liquid away from the air bubbles. As a result, the coating thins, resulting in coalescence and Ostwald ripening.
Although similar events may be observed in any foam system, the high temperature of crema and the way it cools from the top down add another layer of complexity to understanding the physical phenomena that contribute to the development and stability of espresso crème.
According to a prior study, the beverage’s relatively high temperature may hurt foam stability. The idea was that as the water evaporated, the thickness between bubbles decreased, causing the foam to collapse (Navarini et al., 2006).
Another study found that the foam’s lipid amount can alter its stability. Total lipids in a regular espresso (25 mL) range from 45e146 mg for Arabica to 14e119 mg for Robusta (Petracco, 1989; Maetzu et al., 2001).
Because pure Arabica espresso has a more effective overall lipid content than Robusta espresso, the likelihood of lipid-induced froth instability is higher for Arabica.
Because espresso coffee contains emulsified lipids, lipid-induced foam instability could also occur due to oil spreading at the air-beverage interface (Schokker et al., 2002). This aligns with the fact that pure Arabica espresso has a lower surface tension than pure Robusta espresso (Petracco, 2001).
Solid particles, according to studies, have an impact on foam stability. Figure 17.4 depicts such particles in a pure Arabica dry espresso froth. They’re at the plateau’s edge, implying a tendency to be unattached.
Unattached particles primarily follow the net motion of the liquid during drainage, implying that solid particles within the crema play a stabilizing effect. Hydrophobic particles can cause dewetting of the surface by destabilizing the foam through film bridging.
Although the wetting nature of the solid particles found in espresso coffee has yet to be explored, the “tiger skin” effect seen in Arabica espresso could indicate that the solid particles have a foam stabilizing role (also known as the Pickering effect). Antifoaming would otherwise be relatively rapid (Kralchevsky et al., 2002).
However, the differences in foam adhesion found between the two coffee species could be attributed to different liquid-to-dry foam transition rates.
For example, the solid-like appearance of Robusta espresso foam could be due to a faster drainage rate, which appears to be a requirement for adhesion. The rheology of Arabica espresso foam, on the other hand, seems to be liquid-viscous for more extended.
Reference source:
- Primecoffea, Crema trong Espresso và những điều bạn chưa biết, Tháng Chín 30, 2019