
Contraindicated for those who do not like chemistry
Professor Wilhelm Dittmar used the term “alkalinity” to characterize the transitory hardness of water (carbonate hardness). On the other hand, Alkalinity is often mistaken with something else in chemistry. Not everyone who enjoys coffee is also interested in understanding chemistry, so skip the following three lines.
While pH is a measure of the ratio (logarithmic) of OH – free ions to H + ions in solution, total Alkalinity is not; with pH = 7, we have tepid water; when there are many H+ ions, we have an acidic solution (pH7); in contrast, when there are a more significant amount of OH- ions, we have a basic solution (pH>7). This is why lye and total Alkalinity are frequently mistaken (or basic explanation).
Returning to Alkalinity, it is determined by the number of HCO3– ions in the water, which have the power to trap any free H+ ions added to the solution and prevent them from turning it acidic by forming carbonic acid (H2CO3), as seen in the diagram below from 10th grade:
H + + HCO3 – H 2 CO 3
HCO3- is known as an alkaline buffer because of this. Because HCO3- can react with aromatic acids extracted from coffee beans in coffee extract, SCA recommends a total alkalinity range of 40 ppm as CaCO3.
Why is Alkalinity more critical than pH?
Even when rains dropped to the ground, gaseous chemicals such as CO 2, NO 2, and SO 2 diffused into it. Because dissolved CO 2 mixed with water to generate Carbonic acid, rainwater was constantly acidic, with around 5.5. It should not be confused with acid rain, which occurs when the air is contaminated with sulfur and nitrous oxide, resulting in showers with a pH of 4.5 or less.
But why do we need to discuss water acidity? Why not simply follow SCA’s guidelines?
That’s because acid rain is an excellent example of Alkalinity’s influence on pH. One hundred liters of acidic rainfall at pH 4.5 can be neutralized by one liter of regular tap water with an alkalinity of 150 ppm CaCO3.
With water and coffee, the effect of Alkalinity is hundreds of times greater than pH, informing the final acidity of the coffee extract, so the second half of this article will focus on resolving the unfinished business on the pH scale – why alkaline water is better than acidic water and why you should adjust water quality according to ratio or extraction method.
Average tap water is alkaline in the range of 150 ppm CaCO3* and has a total hardness of about 170 ppm CaCO3 – This is considered medium-hard water. This allows tap water to neutralize hundreds of liters of rainwater.
(*) At this point, you might be thinking, “How the hell does CaCO3 go with the weird metric ‘ppm.’ Chemists love to create exotic units of measurement, and this is a prime example. ppm CaCO3 is interpreted as “parts per million CaCO3” (see also 10th-grade chemistry textbook). But because CaCO3 is also used as a unit of measurement for water hardness, it is often confused with Alkalinity.
Illustrate the ‘buffer’ of Alkalinity
For example, in a sunny park, a wooden bench and a stone bench along the path. Even with the sun beaming all day, the wooden chair does not feel too hot, but the stone bench in the same position will be ready to scorch your buttocks.
When you use an infrared thermometer to compare the two chairs, you can tell that the stone bench has a lot of heat trapped in it (because the specific heat capacity of stone is much greater than that of wood). The stone seat will stay warm considerably longer after the sun has set for the same reason.

If you’re sitting on a park bench, you can see if it’s made of stone or not. Meanwhile, the pH of water indicates the existing state of the water; it does not indicate how the pH will alter when coffee is added.
The Alkalinity of the water has a significant impact on this alteration. The acids in the coffee will be significantly neutralized if the water is soaked with a considerable amount of alkali (such as a stone bench that holds a lot of heat). As a result, Alkalinity gives you a signal as to how much taste your coffee will lose when paired with water.
The acidity you get from a given cup of coffee corresponds to the amount of acid extracted from the coffee minus the amount of acid that has neutralized the alkali in the water.
Meaning of alkaline buffer in coffee extract
Understanding the Alkalinity and acidity of the water lays the groundwork for understanding how water and coffee interact to create the extract’s acidic character. There are two aspects to which you must pay attention.
The overall Alkalinity of the water comes first. The stronger the Alkalinity of the water, the better it is in neutralizing the natural acids in coffee (such as citric acid, Quinic Acid, Chlorogenic Acid, etc.). The acidity is still present, but we don’t detect it in the taste; in this situation, it’s referred to as “flat taste.”

The percentage of water in your coffee extraction is the second thing to consider. The coffee ratio in a cup of Ristretto is typically extraction = 11 or 11.5 – The Pour Over approach, on the other hand, raises this ratio to 115 percent.
This means that only 10% of the Alkalinity in intense Espresso is accessible to counteract some of the natural acidity. This is something to think about because, with the same coffee and water, the Espresso will have a higher edge than a pour-over that has been neutralized ten times more alkali.
Extended inference
Continuing to follow this logic (as well as the SCA Standards Committee’s experimental studies), the range of total Alkalinity in water may have to expand to a new frontier.
Instead of being limited to around 40mg/l, Alkalinity in water when brewing Espresso can range between 250 and 450 mg/l; that is, the current total Alkalinity should be multiplied by 7.3 (corresponding to the coefficient between a cup of Espresso (water/coffee ratio = 2)
According to SCA research, when high total hardness water (>250 ppm CaCO3) is used, flavors in coffee are significantly neutralized. When low total hardness water (40 ppm) is used, flavors in coffee are significantly balanced. The taste of CaCO3 is almost unchanged. However, regardless of how complex the water is, experiments demonstrate that total hardness does not affect the overall effectiveness of the extraction (20 to 250 ppm CaCO3). controls the amount of soluble coffee extracted
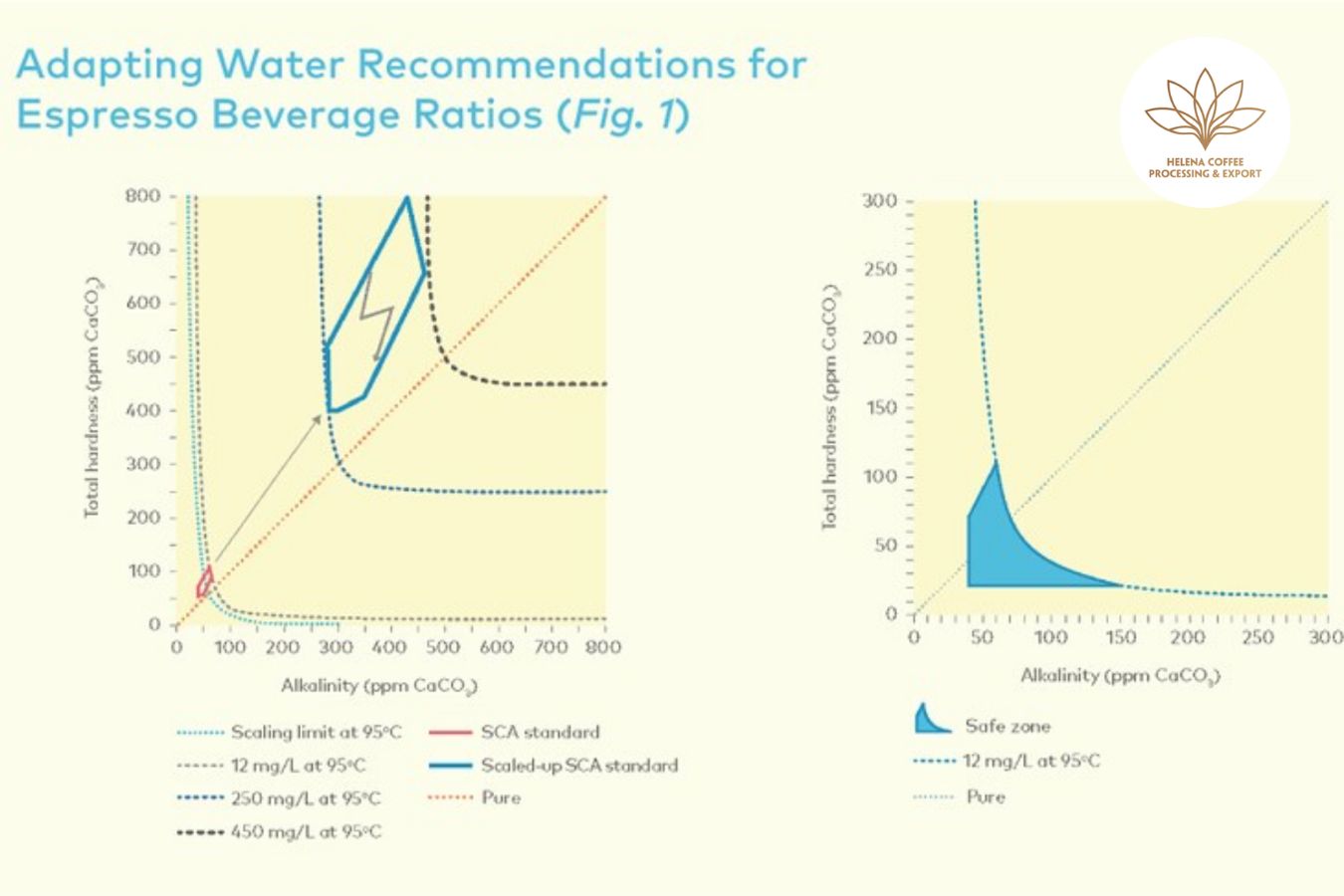
However, the exceptionally high potential for scale development (think 0.25–0.45g salt per liter of water) puts the Alkalinity of espresso brewing water in the “danger” zone. Ingredients in water lead to heavy deposits or corrosion of your apparatus.
The SCA’s coffee water standards document provides critical insights in this piece. Professor DR. MARCO WELLinger – A coffee researcher in chemistry, technology, and sensory analysis at the Institute of Chemistry and Biotechnology at ZHAW Wädenswil – presented his research on Water and Coffee Acidity: How to Adapt Your Water for Different Extraction Methods.
Related Posts:
References:
- Primecoffea, Tác động của độ kiềm đến chiết xuất cà phê, Tháng bảy 6, 2020
