
Let’s find out what essential chemical constituents are in unroasted coffee beans using Bean In Love within the confines of this article!
The following is a list of organic compounds
Carbohydrates
Carbohydrates (hydrated carbon molecules with complex structures) make up roughly 40-50 percent of the chemical makeup of coffee and are split into two categories:
The central element in coffee beans is cellulose, which comprises high-molecular-weight carbohydrates (also known as polysaccharides). This is the critical component that gives coffee its structure and form. Hundreds of chemical elements reside in this structure, which will alter, separate, and combine to form the distinctive chemicals of roasted coffee.
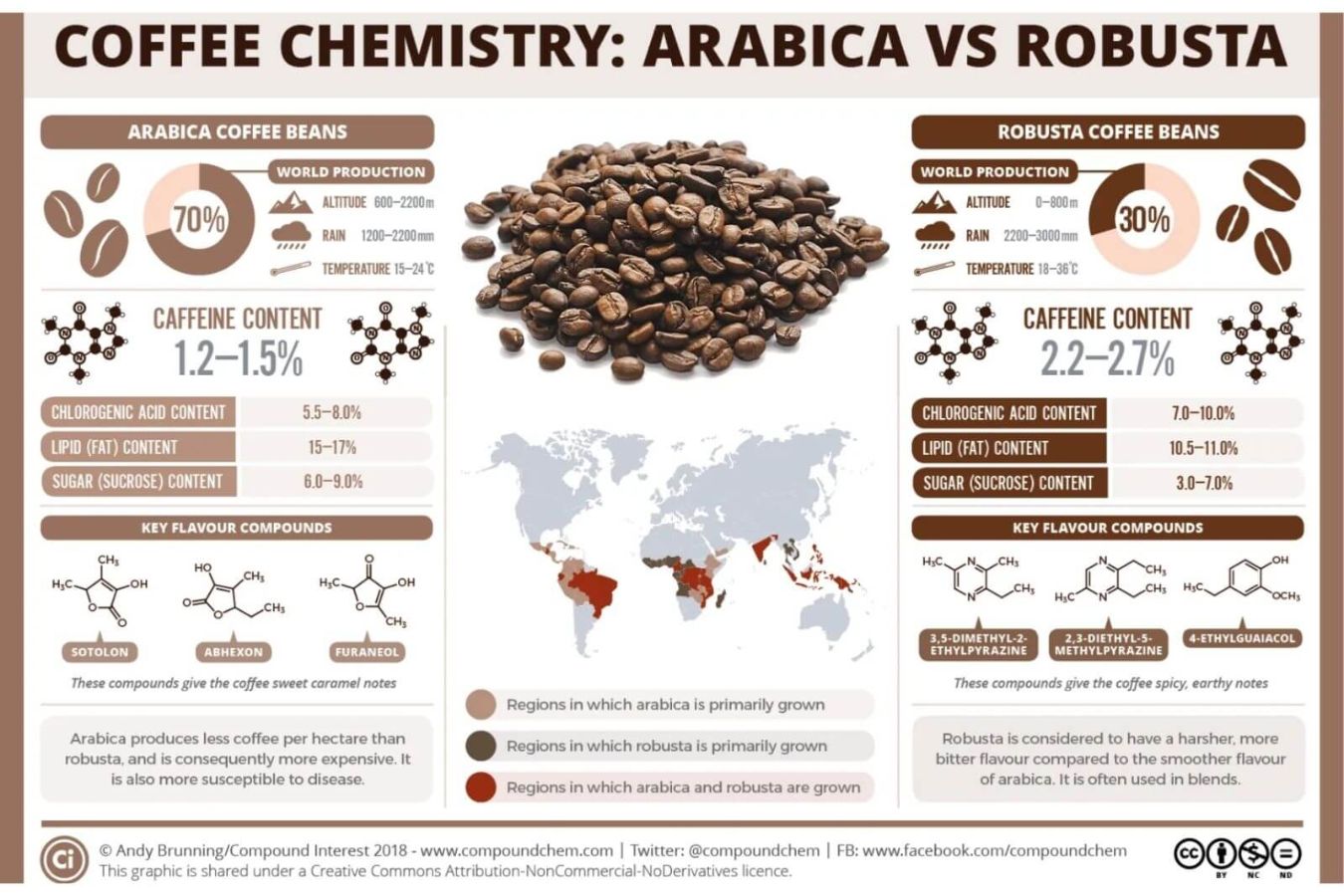
Sucrose is a low molecular weight carbohydrate (commonly known as sugar) found primarily in green coffee. Sucrose makes up 6-9 percent of green coffee’s weight (Arabica).
This amount of sugar is determined by the coffee variety, growing region, soil, and, most importantly, the ripeness of the coffee berry at harvest. During the browning and caramelization stages of the roasting process, the amount of sugar in the fruit plays a vital part in providing sweetness for the cup of coffee and the distinctive flavor of the coffee.
Protein is a type of food
Protein makes up roughly 10% to 13% of the dry weight of green coffee. The protein composition contains the primary amino acids cysteine, alanine, phenylalanine, methionine, and proline are found in the protein composition…
During the roasting process (by the Maillard reaction), these amino acids and sugars will mix to form Glycosylamines and Melanoidins, which give coffee its bitter flavor, brown color, and roasted aroma.
Organic acids are a type of organic acid
Chlorogenic acids (CGAs), citric, quinic, caffeic, malic, acetic, and formic acids are organic acids. CGAs are essential organic acids that makeup roughly 7-10% of the weight of green coffee beans.
CGAs cause coffee’s bitter flavor, also anti-insect and anti-pest compounds. Because Robusta coffee contains more CGAs (9.9%) than Arabica coffee (8.1%), Robusta coffee has a more bitter flavor and is more resistant to pests and viruses. CGA has a strong antioxidant capacity.
Lipids are a type of lipid (Fat)
Triglycerides are the most common type of fat found in coffee. Because fat is insoluble in water, it lingers in coffee after brewing, especially without a filter. The fat in coffee aids in the preservation of fragrance molecules (aroma) and creates a sense of fullness when consumed.
Arabica coffee has a high-fat content (15-17%), while Robusta has a low-fat range (10.5-11 percent ). You will gain more fat if you drink high-quality coffee. When roasted seeds are stored poorly, fat causes them to oxidize faster and emit a foul odor.
Alkaloids
Caffeine and trigonelline are two of the most common alkaloids (organic chemicals with at least one nitrogen atom) found in coffee beans. Coffee’s bitter flavor and stimulating qualities are due to these alkaloids, which make up around 1% of the bean’s weight.
Caffeine is responsible for around 10% of the bitter taste of coffee, but it also accounts for the majority of the stimulating impact. To combat insects, coffee plants produce their caffeine. Robusta coffee has a higher caffeine concentration than Arabica, making it more pest resistant. The roasting procedure of coffee does not increase or decrease the caffeine level of the bean, contrary to popular perception.
Trigonelline is the bitterest component of coffee. Trigonelline decomposes (around 218 degrees Celsius) during roasting to produce a variety of aromatic chemicals and breaks down into pyridines and nicotinic acid (also known as vitamin B3).
Coffee’s aromatic compounds
More than 200 aromatic chemicals are found in green coffee beans, all of which have the physical property of being volatile. Under the action of heat, these chemicals separate, mix, and produce over 800 additional fragrant compounds when coffee is roasted. This is the fundamental reason behind coffee’s appealing and distinctive scent.

Acids, aldehydes, ketones, alcohols, phenols, esters, and other constituent molecules make up complex aromatic compounds. Because aromatic components are very volatile and easily oxidized, coffee can lose flavor and quality if not stored properly.
Minerals
Phosphorus, chlorine, magnesium, potassium, nitrogen, copper, aluminum are the most common components that give the coffee an awful taste. Minerals make up roughly 3-4 percent of the total—the lesser the mineral level, the greater the coffee quality, and vice versa.
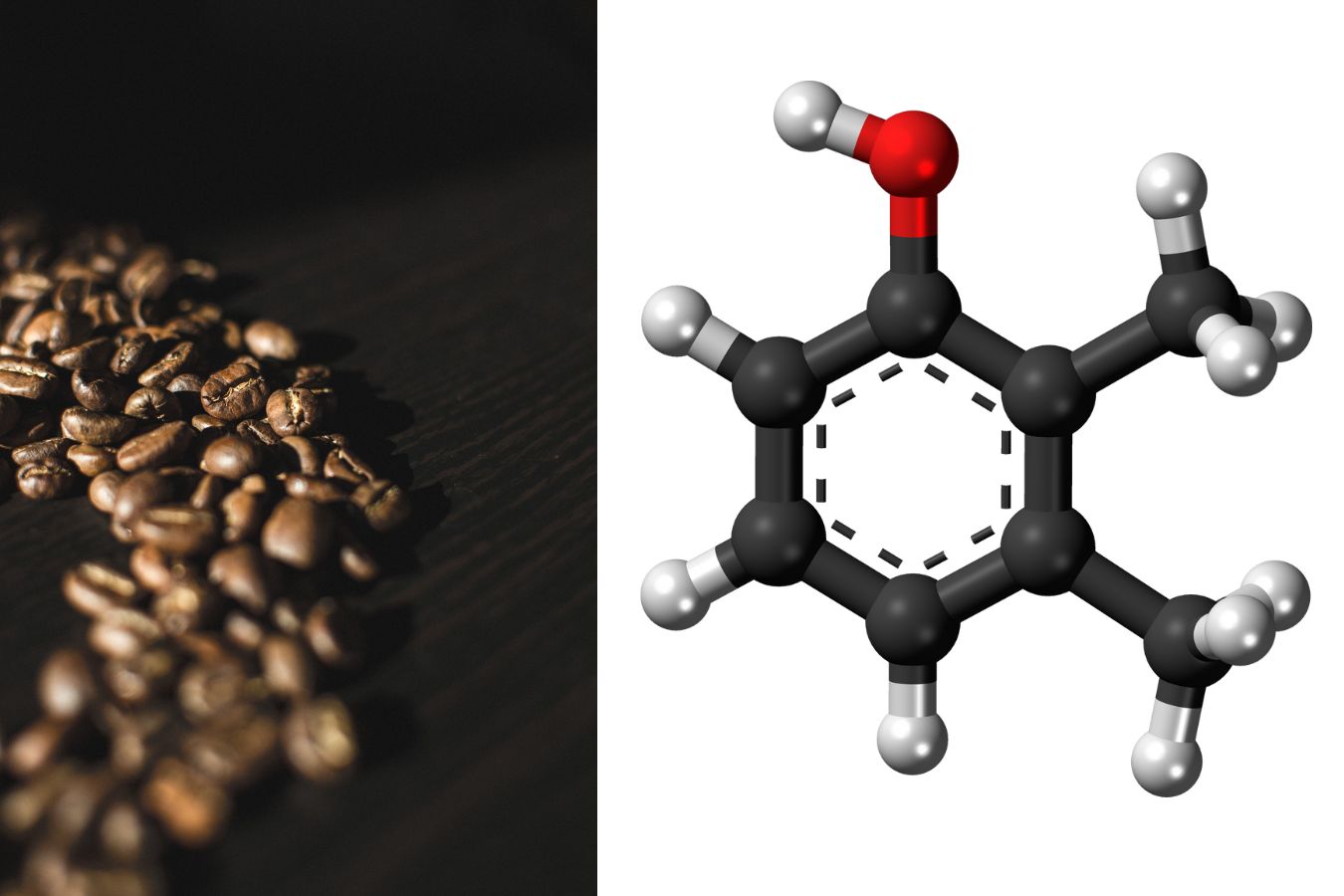
Caffeine
Caffeine (1,3,7-trimethylxanthine) is the most well-known N compound due to its physiological effects (stimulation of the central nervous system, increased blood circulation, and respiration). It has a mild bitter taste. A coffee drink contains 10% caffeine and 6% chlorogenic acid.
Caffeine levels in beans are reduced during roasting. The pharmaceutical and soft drink industries use synthetic caffeine and caffeine obtained through decaffeination. Synthetic caffeine is produced by methylating xanthine derived from uric acid and formamide.
Caffeine is used as a CNS stimulant in medicine, usually in combination with another therapeutic agent and in analgesic preparations. Theobromine has diuretic and smooth muscle relaxant properties, but it is not commonly used. Theophylline is a smooth muscle relaxant that is frequently prescribed in long-term formulations to reduce side effects.
It is also available in the form of aminophylline (a more soluble preparation containing theophylline and ethylenediamine) and choline theophyllinate (theophylline and choline). The alkaloids can be isolated from natural sources or synthesized completely or partially [5].
Caffeine, theobromine, and theophylline are examples of purine alkaloids, as shown in Figure 1. As alkaloids, they have a limited distribution, but their origins are very similar to those of purine bases like adenine and guanine, which are fundamental components of nucleosides, nucleotides, and nucleic acids.
Caffeine is the most widely consumed and socially accepted natural stimulant and primarily consumed in beverages such as tea, coffee, and cola. When compared to caffeine, theophylline is far more important as a drug compound due to its muscle relaxant properties, which are used to treat bronchial asthma. Theobromine is the primary component of cocoa and related chocolate products.
Reference source:
- COFFEE Recent Developments; Book by R.J. Clarke and O.G. Vitzthum
- www.science.org.au/ Brew up some coffee chemistry
- www.edwinvanbloois.com/ Biochemistry of coffee
- www.coffeechemistry.com/ Joseph A. Rivera served as the former Director of Science & Technology for the Specialty Coffee Association of America.